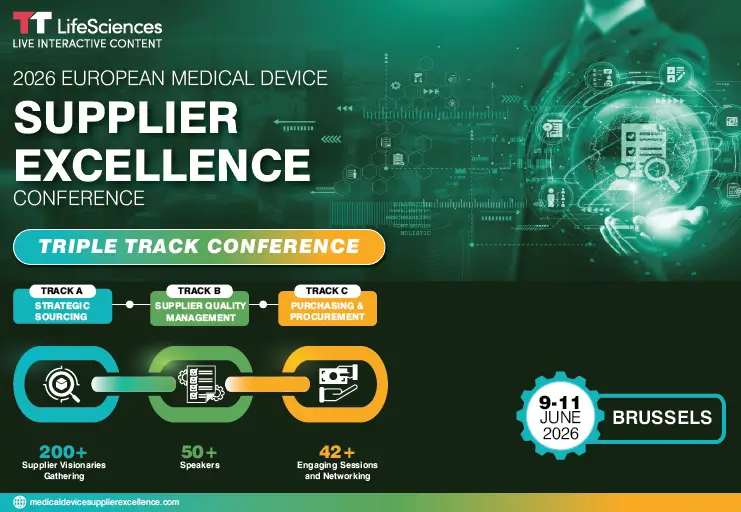
2026 EUROPEAN MEDICAL DEVICE SUPPLIER EXCELLENCE CONFERENCE
Brussels | 2026
End-to-End Supplier Excellence: From Driving Strategy, Quality & Operational Impact
key information
- Brussels
- 9th – 11th June 2026
#TTSE & #TTSUPPLIEREXCELLENCE
Conference Insights
Step inside Europe’s most influential Medical Device Supplier Excellence Conference, a 3-day journey redefining how MedTech builds trust, transparency, and resilience across its supply chains.
Immersive Workshops:
- Immersive Workshops: Real-world simulations on supplier risk mapping, digital scorecards, and agile sourcing frameworks.
- Thought-Provoking Panels: Industry leaders decoded MDR & IVDR challenges, ESG expectations, and next-gen sourcing models.
- Visionary Fireside Chats: Trailblazers shared candid lessons on transforming procurement into a strategic powerhouse.
- Collaborative Energy: Speed networking, interactive roundtables, and the final plenary on “Future-Proofing Supply Chains” ignited ideas and inspired lasting change.
3 DAYS OF INSIGHTS & INNOVATION
DAY 1 – WORKSHOP DAY
- Track A – Strategic Sourcing Excellence: Supplier Risk Mapping & Dual Sourcing Strategy / Digital Transformation with AI, Data & Dashboards
- Track B – Supplier Quality & Audit Readiness: Advanced Risk Assessment & SCAR Optimization / Designing High-Impact Supplier Scorecards
- Track C -Purchasing & Procurement Operations: Value-Based Procurement / Digital Tools & Contract Compliance for End-to-End Traceability
- Exclusive Workshop Challenge: Collaborative problem-solving in breakout groups to create real-world supplier solutions.
DAY 2 – CONFERENCE DAY
- Designing Global & Regional Sourcing Strategies for Resilience
- APQP & PPAP Implementation
- From Transactional Purchasing to Strategic Supplier Partnerships: A Manufacturer’s Journey
- Roundtable Discussions: SCAR Decision Frameworks: To SCAR or Not to SCAR
- Joint Plenary Panel: The Regulator’s View – EU MDR, IVDR, ESG & AI in Supplier Oversight
DAY 3 - CONFERENCE DAY
- Supplier Innovation Programs & Co Development Governance
- Navigating ESG Compliance in MedTech Procurement: Legal Risks and Opportunities
- Fireside Chat: Balancing Domestic vs. Global Sourcing Post-COVID, Ukraine & Geopolitical Conflicts
- Joint Plenary Panel: Future-Proofing Supply Chains – What Still Needs to Change?
- Case Study – Underperforming Suppliers – Intervention and Recovery Strategies
Our Industry Specialists

Eric Marino

Andy Walker

Laurent Linden

Geraldine Gough

Suchit Gupta

Marta Carnielli

Carine Cochereau

Geraldine Gough

David Festa

Péter Császár

Khalid Dib

Jan Krch

Mary Ryan

Matthew Savage

Vishwas Sharma

Izabela Banas

Davide Caon
Ana Cukalovic
WHY TAKE PART
The 2026 European Medical Device Supplier Excellence Conference is where Europe’s sourcing, procurement, and supplier quality leaders come together to elevate partnerships, drive resilience, and set new benchmarks for excellence. A rare opportunity to collaborate, problem-solve, and future-proof supply chains with the people shaping the future of medical device supplier success.
Connect with Europe’s top procurement, sourcing, and supplier quality leaders to exchange insights, benchmark practices, and gain multidisciplinary perspectives.
Participate in workshops, roundtables, and interactive sessions to tackle real-world challenges with experts and peers to learn how to navigate global risks, regulatory shifts, and operational complexities to drive excellence.
Gain insights from regulators and global agencies on ESG, supplier oversight, and risk management.
Explore AI, dashboards, and data-driven strategies that optimize supplier performance and procurement efficiency.
Connect with peers, suppliers, and experts to strengthen collaboration and future-proof your supply chain
KEY TAKEAWAYS
DESIGN FOR RESILIENCE
Build agile global and regional sourcing strategies that withstand disruption and ensure continuity.
DRIVE VALUE-BASED PROCUREMENT
Align purchasing decisions with patient outcomes, total cost efficiency, and long-term value creation.
EMPOWER QUALITY WITH AI
Transform supplier monitoring—from dashboards to predictive audits—using data and automation.
BALANCE ESG WITH PERFORMANCE
Integrate sustainability and ethical sourcing into supplier evaluation without compromising quality or cost.
NAVIGATE NMPA READINESS
Equip suppliers for China’s regulatory expectations and strengthen global market access.
FUTURE-PROOF SUPPLY CHAINS
Redefine resilience, collaboration, and technology adoption to stay ahead of evolving global challenges.
Moments Captured
Take a glimpse into the highlights of our conferences, where ideas come to life and connections are made
Our Testimonials



Perfect networking opportunity and learning about alternative ways to handle known topics.
– Supplier Quality Manager

The event brought together insightful discussions and valuable perspectives on key topics, such as PPAP, supplier collaboration, balancing quality and cost, risk-based approaches, and best practices in new product development & supplier quality management.
– Supplier Quality Manager

A great networking opportunity with an amazing variety of speakers and attendees. Highly recommended
– Senior Quality Engineer

Great opportunity to discuss strategies to successfully manage supply chain challenges.
– Head of Certification IVD

As usual, very interesting and insightful.
– Senior Director Supplier Quality Management

Inspiring talks and exchanges with peers.
– External partner manager

Best live attending to get the maximum experience.
– Purchasing manager

Great possibilities for networking and personal exchange of experiences due to sufficient time reserved during the conference.
– Professor

Opportunity for engaging with the Notified body & Industry leaders.
– External Contract Mfg. & Supplier Quality Lead
YOUR ALL-IN-ONE GUIDE TO THE EVENT
Get the full scoop on what’s in store!
Explore the event overview and dive into the agenda for the 2026 EUROPEAN MEDICAL DEVICE SUPPLIER EXCELLENCE CONFERENCE—your gateway to 3 days of strategy, science, and innovation.
Our Sponsors
Platinum Sponsors
COLLOCATE WITH US/EUROPE
- 20th - 22nd October 2026
- Boston, MA, US
You're In Good Company